Introduction:
Bone and tissue transplantation represent groundbreaking advancements in medical science, offering hope and improved quality of life for individuals with various orthopaedic and reconstructive needs.
These procedures involve the transfer of bone, cartilage, tendons, ligaments, and other tissues from one part of the body to another or from a donor to a recipient.
From repairing damaged joints and reconstructing facial features to restoring mobility and function, bone and tissue transplantation techniques have revolutionised the field of orthopaedic surgery and plastic surgery.
Over the years, significant strides have been made in refining transplantation procedures, enhancing surgical techniques, and improving patient outcomes.
With ongoing research and innovation, surgeons continue to push the boundaries of what is possible, exploring new approaches to transplantation and developing novel biomaterials to support tissue regeneration and integration.
The Importance of the Musculoskeletal System for UK Residents
The musculoskeletal system, consisting of muscles, bones, joints, tendons, and ligaments, is indispensable for facilitating daily tasks and movements among individuals residing in the United Kingdom.
Musculoskeletal disorders and diseases profoundly impact the quality of life within the UK population.
These conditions rank among the most commonly reported impairments in the country, affecting over 100 million individuals.
Chronic and permanent limitations in function due to musculoskeletal conditions are prevalent, resulting in substantial societal costs exceeding £800 billion annually.
However, the repercussions extend beyond financial implications.
Constraints on physical activity, persistent or severe pain, visible deformities, or the inability to perform daily tasks commonly all contribute to a diminished quality of life for affected individuals.
These challenges underscore the critical importance of maintaining musculoskeletal health and addressing issues promptly to mitigate their impact on the well-being of residents in the United Kingdom.
Restoring Musculoskeletal Health
Orthopaedics is a specialised medical discipline dedicated to both diagnosing and treating conditions affecting the musculoskeletal system. Within this field, orthopaedic surgeons, also referred to as orthopaedists, undergo extensive training to proficiently utilise medical, physical, and surgical interventions for addressing injuries or diseases impacting musculoskeletal function.
The path to becoming an orthopaedic surgeon involves rigorous education and training, ensuring a high level of expertise and competence.
Throughout this journey, orthopaedic surgeons acquire a diverse skill set in various medical and surgical techniques, enabling them to perform procedures effectively.
With their comprehensive training and commitment to patient care, orthopaedic surgeons strive to restore function and improve the quality of life for individuals affected by musculoskeletal conditions.
Their dedication contributes significantly to the advancement of orthopaedic surgery and the enhancement of musculoskeletal health outcomes.
Types of Grafts Utilised in Orthopaedic Surgery
Orthopaedic surgeons utilise various grafts in surgical procedures to address musculoskeletal issues. These grafts include autografts, which involve transplanting bone or tissue from one part of a person’s body to another, and allografts, which involve transplanting bone or tissue from one person to another.
Allograft musculoskeletal tissue is typically sourced from individuals who have chosen to donate their organs and tissues upon their passing.
These generous donors, often in good health before their unexpected demise, provide a valuable resource for allografts. In some cases, allograft bone may also be sourced from living patients.
The term “graft” encompasses both autografts and allografts and is commonly used in orthopaedic surgery to denote tissue or bone transplantation procedures.
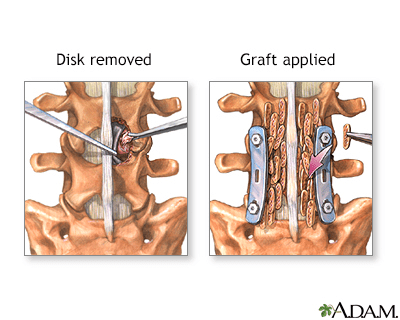
The Spinal Bone Graft Series
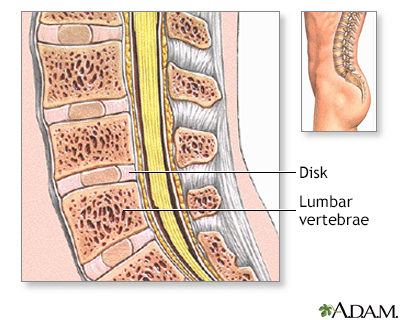
Normal Anatomy
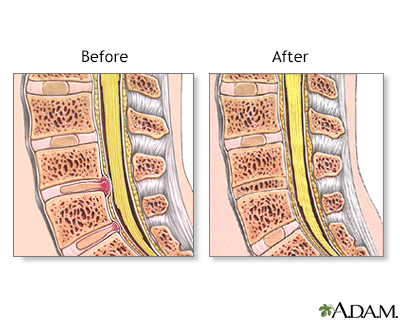
The spinal vertebrae are interspersed with cartilage disks containing a gel-like substance, serving to cushion the spinal column.
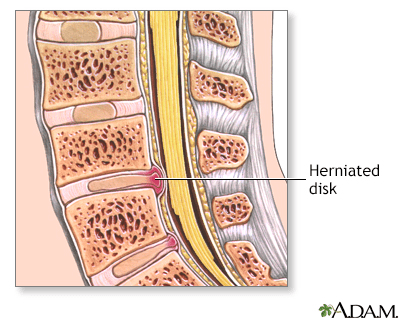
Indications for Herniated Disk
A herniated disk occurs when the soft, gel-like centre of an intervertebral disk (nucleus pulposus) protrudes through a weakened area of the disk.
This condition could lead to symptoms such as back pain and irritation of the nerve roots.
Procedure Overview: Part 1
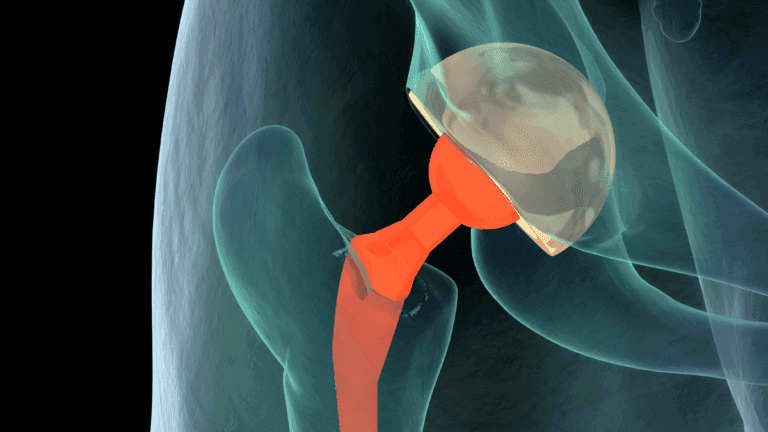
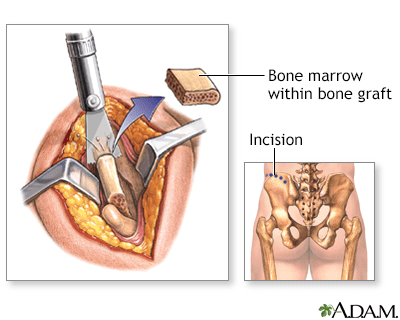
The hip bones are an excellent source for bone grafts due to their rapid regeneration and limited weight-bearing function.
Under general anaesthesia, to ensure complete pain relief and unconsciousness, an incision is made along the crest of the hip bone. A segment of bone is then carefully extracted before the incision is closed with stitches (sutures). Some discomfort may be experienced in the vicinity of the incision site.
Procedure Overview: Part 2
The bone graft addresses bone defects or facilitates the fusion of fractures or joints. An incision is made directly over the affected bone area, and the bone graft is carefully shaped and inserted into the defect. To secure the graft in place, pins, plates, or screws may be employed. Following graft placement, the incisions are closed with stitches (sutures). A splint or cast is typically applied to safeguard against injury or movement during the healing process.
Postoperative Care
The majority of bone grafts yield successful outcomes in facilitating bone defect healing, with minimal risk of graft rejection. However, vigorous physical activity is typically restricted for a period of up to three months following the procedure.
Bone Recovery Process from Donors in the UK
Tissue banks in the United Kingdom play a vital role in retrieving, processing, and distributing allograft tissue, including bone. These banks specialise in donor screening, recovery, and storage, ensuring that donated musculoskeletal tissues are handled carefully. Trained transplantation specialists oversee the recovery and processing of donated tissues, undergoing rigorous training and certification to maintain proficiency in tissue banking practices.
While the UK may not have an organisation equivalent to the American Association of Tissue Banks (AATB), regulatory bodies such as the Human Tissue Authority (HTA) oversee tissue banking operations. The HTA is responsible for licensing and regulating tissue banks, ensuring compliance with established standards and guidelines for human tissue’s safe and ethical use. Additionally, tissue banks in the UK may opt for voluntary accreditation through organisations like the European Association of Tissue Banks (EATB) to uphold industry standards and enhance the quality and safety of donated tissues.
Screening Process for Potential Donors
Prior to donation, rigorous screening procedures are conducted to ensure the safety and suitability of donated tissues.
This includes a thorough physical examination, a comprehensive medical history assessment, and a review of social risk factors.
In the United Kingdom, potential donors undergo similar screening processes, which involve collecting detailed medical histories and assessing social risk backgrounds to identify individuals with high-risk behaviours.
The information gathered from potential donors is compared against criteria established by regulatory bodies such as the Human Tissue Authority (HTA) in the UK, as well as standards set by international organisations.
All donated tissues are held in quarantine until microbiological and blood tests are completed.
These tests, required by regulatory authorities like the HTA (Human Tissue Authority )and the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK, include analysis for infectious diseases like HIV, hepatitis B and C, and syphilis.
A team of medical specialists, including infectious disease experts and tissue banking professionals, evaluates the screening and testing information to ensure the safety of the donated tissues.
. No allograft can be released for transplantation until the tissue bank’s medical director or designated authority confirms that the tissue meets safety standards following a thorough review of the screening and testing results.
Safety of Tissue Transplantation
Preparing tissue for transplantation begins with meticulously removing debris and organic matter and soaking in various solutions to prevent bacterial and viral transmission.
Processing and packaging occur in clean room environments under sterile conditions to preserve biological integrity.
In some cases, low-dose radiation may be utilised to aid in sterilisation. According to United States Pharmacopeia (USP) guidelines, final processed tissues undergo rigorous microbiological testing to ensure compliance with regulatory standards.
While there is a theoretical risk of disease transmission, allografts subjected to stringent donor screening, serological testing, and formal processing have substantially minimised this risk.
The (FDA) US Food and Drug Administration (FDA) has closely regulated this field, ensuring the safety of allograft transplants since 1993. In the UK, regulatory bodies such as the Human Tissue Authority (HTA) and also the Medicines and Healthcare Products Regulatory Agency (MHRA) oversee tissue transplantation practices to uphold safety standards and minimise patient risks.
Over the past decade, millions of musculoskeletal allografts have been distributed to surgeons in the UK, demonstrating a remarkable safety record in tissue transplantation.
Post-Transplantation Outcome
Following transplantation, the bone or soft tissue graft undergoes a process where it gradually becomes integrated into the recipient’s body. Over time, the transplanted tissue is transformed into new living bone or soft tissue, seamlessly assimilating with the surrounding anatomy to form a functional unit.
Ensuring Safety and Covering Costs in Tissue Banking
In the United Kingdom, the costs associated with recovering organs and tissues for donation are not transferred to the donor or their family, as it is illegal buying and selling human organs or tissues.
Tissue banks in the UK adhere to stringent protocols to ensure safety, which includes maintaining professional expertise in donor screening, testing, and quality control. They invest in state-of-the-art processing procedures and stay updated on advancements in tissue sterilisation and graft incorporation techniques.
They continuously monitor the development of new products, processing procedures, and regulatory requirements set forth by authorities like the Human Tissue Authority (HTA) and the Medicines and Healthcare Products Regulatory Agency (MHRA).
These efforts entail costs associated with personnel and services involved in acquiring, processing, and storing allografts. While costs are kept to a minimum, they are necessary to uphold safety standards and ensure the utmost safety in tissue transplantation within the UK.
These expenses are typically covered by hospitals, surgeons, or recipients as part of the transplantation process.
Advancing Research and Regulation in Tissue Banking:
In the United Kingdom, tissues not meeting human-use transplantation criteria are directed towards research endeavours to propel musculoskeletal transplantation science forward. Additionally, selected tissues may be allocated to tissue engineering programs aimed at innovation.
Regulatory oversight in the UK is overseen by the Human Tissue Authority (HTA), which ensures compliance with stringent standards outlined in the Human Tissue (Quality and Safety for Human Application) Regulations 2007.
These regulations establish national benchmarks for safety and quality in tissue banking practices.
The HTA, akin to the American Association of Tissue Banks (AATB) in the United States, functions as the competent authority, issuing expected standards and codes of practice to tissue establishments. These establishments must undergo rigorous inspections to maintain accreditation, guaranteeing adherence to safety protocols and quality standards.
Collaboration between the HTA and regulatory bodies, such as the Medicines and the Healthcare Products Regulatory Agency (MHRA), further strengthens oversight and ensures the safety of tissue transplantation procedures in the UK.
Research in musculoskeletal transplantation is also supported by organisations like the British Orthopaedic Association (BOA), which advocates for using allografts in appropriate patient populations, contributing to advancements in orthopaedic care and treatment outcomes.